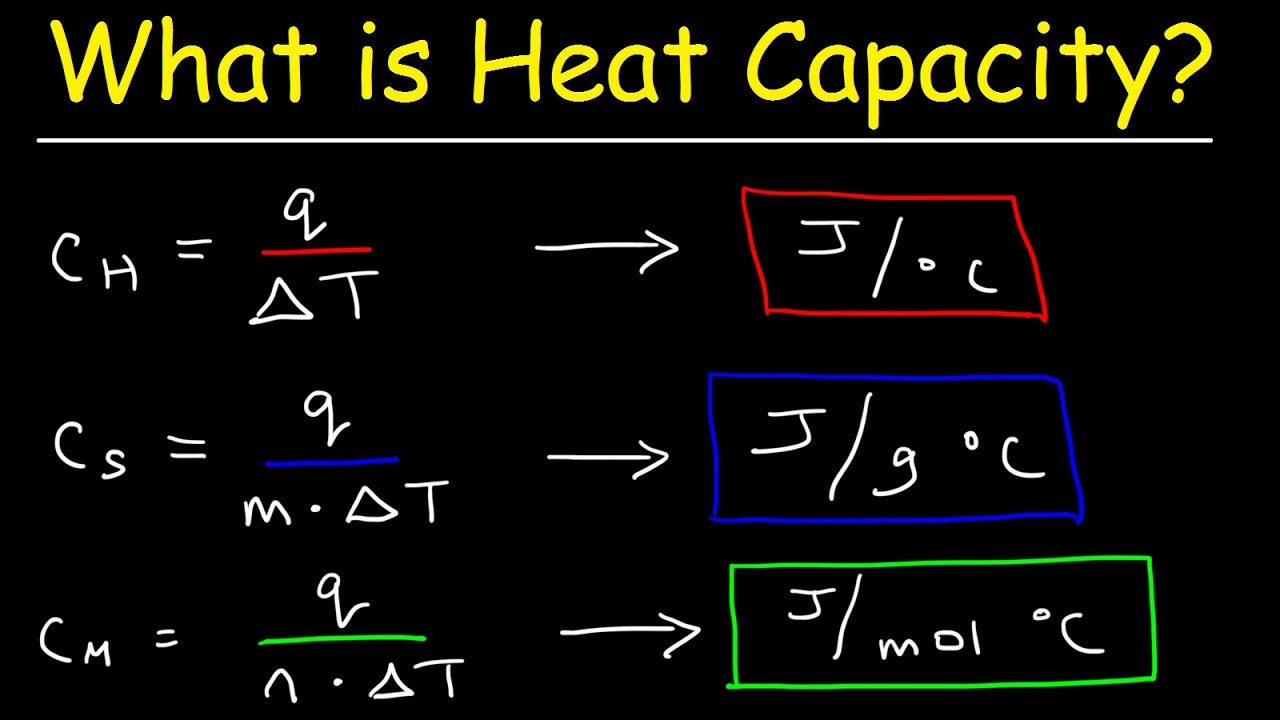
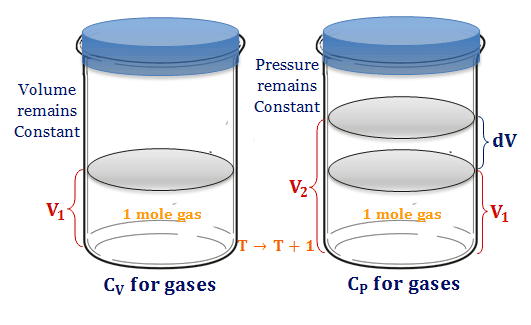
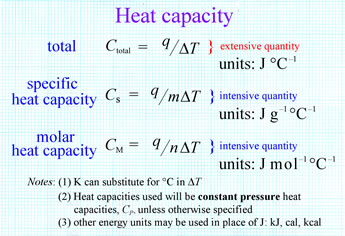
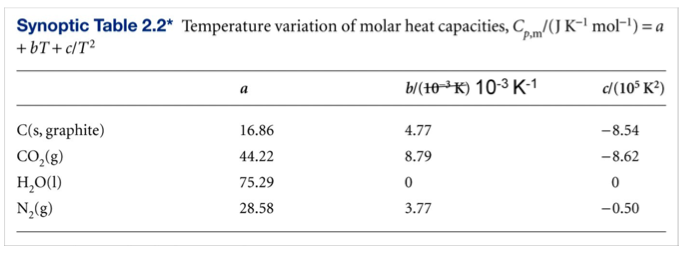
Find the molar heat capacity in a process of a diatomic gas if it does a work of Q/4 when a heat of Q is supplied to it

Explain heat capacity in detail Molar heat capacity, specific heat capacity, Cp and Cv (definition and ratios) - Chemistry - Thermodynamics - 9081453 | Meritnation.com
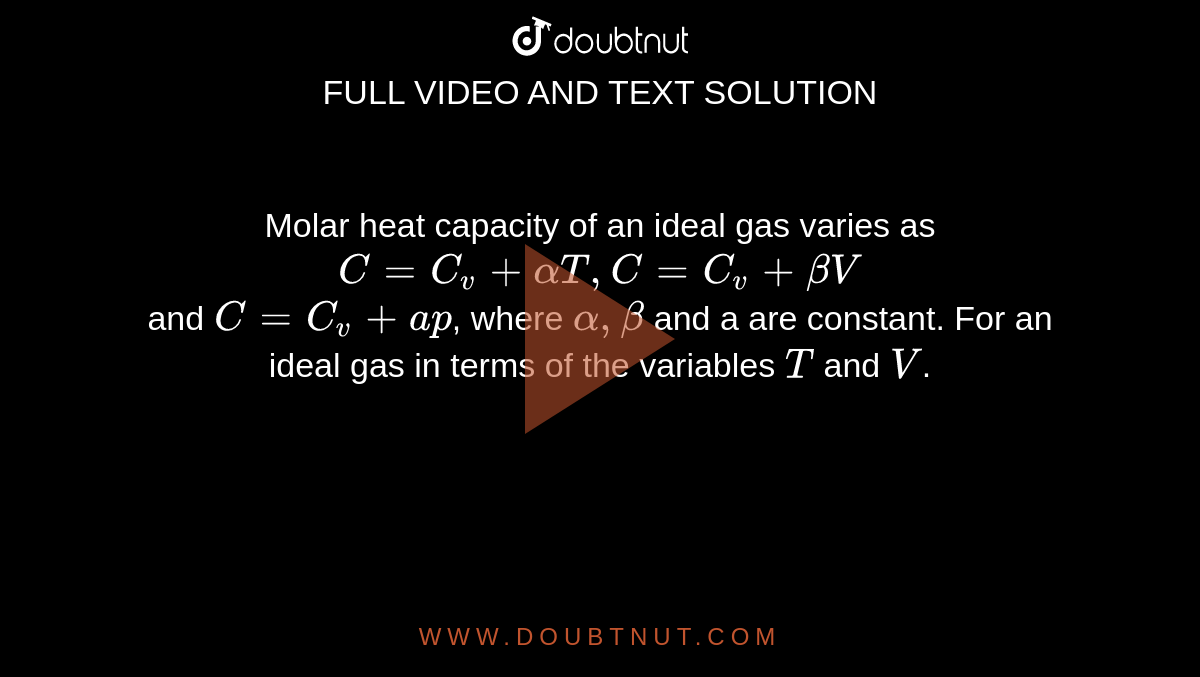
Molar heat capacity of an ideal gas varies as C = C(v) +alphaT,C=C(v)+betaV and C = C(v) + ap, where alpha,beta and a are constant. For an ideal gas in terms of

Standard molar heat capacity of water: filled circle literature values... | Download Scientific Diagram
![Plot of the molar heat capacity of [Fe(2-pic)3]Cl2‚MeOH vs temperature.... | Download Scientific Diagram Plot of the molar heat capacity of [Fe(2-pic)3]Cl2‚MeOH vs temperature.... | Download Scientific Diagram](https://www.researchgate.net/publication/11899657/figure/fig1/AS:590187696500736@1517723120655/Plot-of-the-molar-heat-capacity-of-Fe2-pic3Cl2MeOH-vs-temperature-The-dotted-and.png)
Plot of the molar heat capacity of [Fe(2-pic)3]Cl2‚MeOH vs temperature.... | Download Scientific Diagram
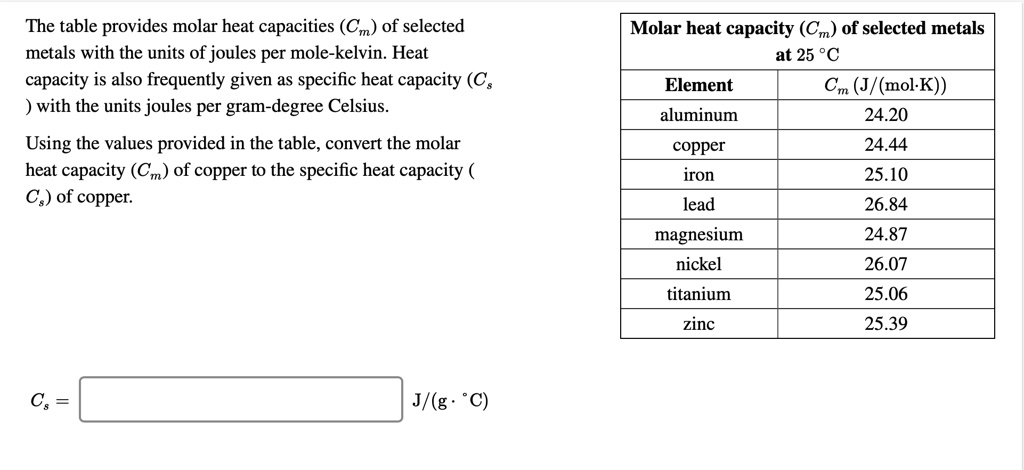
SOLVED: The table provides molar heat capacities (Cm) of selected metals with the units of joules per mole-kelvin. Heat capacity is also frequently given as specific heat capacity (C; ) with the

The molar heat capacity in a process of a diatomic gas if it does a work of Q/4 when a heat of Q is supplied to it is